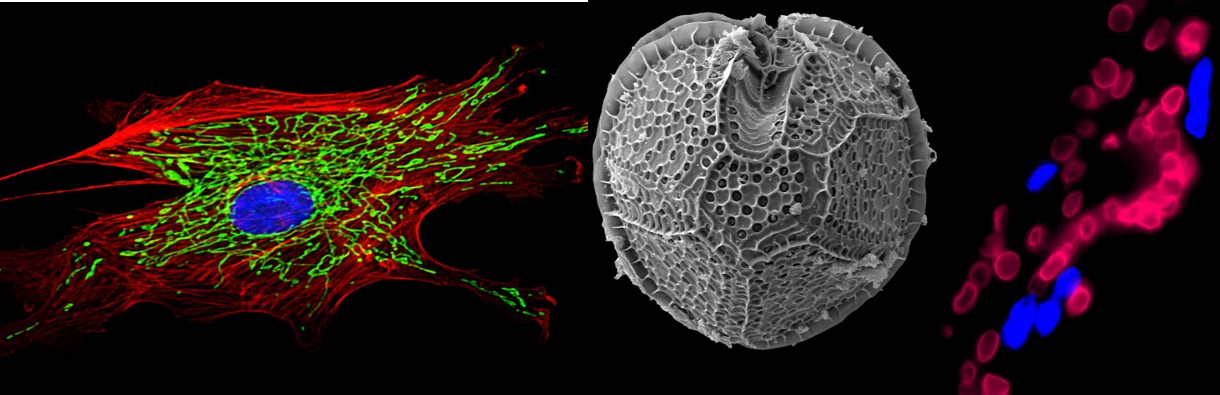
In January 2014, the two papers “Stimulus-triggered fate conversion of somatic cells into pluripotency” and “Bidirectional developmental potential in reprogrammed cells with acquired pluripotency” published in the journal Nature by Haruko Obokata and colleagues took the world of stem cell research by surprise.
Since Shinya Yamanaka’s landmark discovery that adult skin cells could be reprogrammed into embryonic-like induced pluripotent stem cells (iPSCs) by introducing selected embryonic genes into adult cells, laboratories all over the world have been using modifications of the “Yamanaka method” to create their own stem cell lines. The original Yamanaka method published in 2006 used a virus which integrated into the genome of the adult cell to introduce the necessary genes. Any introduction of genetic material into a cell carries the risk of causing genetic aberrancies that could lead to complications, especially if the newly generated stem cells are intended for therapeutic usage in patients.
Researchers have therefore tried to modify the “Yamanaka method” and reduce the risk of genetic aberrations by either using genetic tools to remove the introduced genes once the cells are fully reprogrammed to a stem cell state, introducing genes without non-integrating viruses or by using complex cocktails of chemicals and growth factors in order to generate stem cells without the introduction of any genes into the adult cells.
The papers by Obokata and colleagues at the RIKEN center in Kobe, Japan use a far more simple method to reprogram adult cells. Instead of introducing foreign genes, they suggest that one can expose adult mouse cells to a severe stress such as an acidic solution. The cells which survive acid-dipping adventure (25 minutes in a solution with pH 5.7) activate their endogenous dormant embryonic genes by an unknown mechanism. The researchers then show that these activated cells take on properties of embryonic stem cells or iPSCs if they are maintained in a stem cell culture medium and treated with the necessary growth factors. Once the cells reach the stem cell state, they can then be converted into cells of any desired tissue, both in a culture dish as well as in a developing mouse embryo. Many of the experiments in the papers were performed by starting out with adult mouse lymphocytes, but the researchers also found that mouse skin fibroblasts and other cells could also be successfully converted into an embryonic-like state using the acid stress.
My first reaction was incredulity. How could such a simple and yet noxious stress such as exposing cells to acid be sufficient to initiate a complex “stemness” program? Research labs have spent years fine-tuning the introduction of the embryonic genes, trying to figure out the optimal combination of genes and timing of when the genes are essential during the reprogramming process. These two papers propose that the whole business of introducing stem cell genes into adult cells was unnecessary – All You Need Is Acid.
This sounds too good to be true. The recent history in stem cell research has taught us that we need to be skeptical. Some of the most widely cited stem cell papers cannot be replicated. This problem is not unique to stem cell research, because other biomedical research areas such as cancer biology are also struggling with issues of replicability, but the high scientific impact of burgeoning stem cell research has forced its replicability issues into the limelight. Nowadays, whenever stem cell researchers hear about a ground-breaking new stem cell discovery, they often tend to respond with some degree of skepticism until multiple independent laboratories can confirm the results.
My second reaction was that I really liked the idea. Maybe we had never tried something as straightforward as an acid stress because we were too narrow-minded, always looking for complex ways to create stem cells instead of trying simple approaches. The stress-induction of stem cell behavior may also represent a regenerative mechanism that has been conserved by evolution. When our amphibian cousins regenerate limbs following an injury, adult tissue cells are also reprogrammed to a premature state by the stress of the injury before they start building a new limb.
The idea of stress-induced reprogramming of adult cells to an embryonic-like state also has a powerful poetic appeal, which inspired me to write the following haiku:
The old warrior
plunges into an acid lake
to emerge reborn.
(Read more about science-related haikus here)
Just because the idea of acid-induced reprogramming is so attractive does not mean that it is scientifically accurate or replicable.
A number of concerns about potential scientific misconduct in the context of the two papers have been raised and it appears that the RIKEN center is investigating these concerns. Specifically, anonymous bloggers have pointed out irregularities in the figures of the papers and that some of the images may be duplicated. We will have to wait for the results of the investigation, but even if image errors or duplications are found, this does not necessarily mean that this was intentional misconduct or fraud. Assembling manuscripts with so many images is no easy task and unintentional errors do occur. These errors are probably far more common than we think. High profile papers undergo much more scrutiny than the average peer-reviewed paper, and this is probably why we tend to uncover them more readily in such papers. For example, image duplication errors were discovered in the 2013 Cell paper on human cloning, but many researchers agreed that the errors in the 2013 Cell paper were likely due to sloppiness during the assembly of the submitted manuscript and did not constitute intentional fraud.
Irrespective of the investigation into the irregularities of figures in the two Nature papers, the key question that stem cell researchers have to now address is whether the core findings of the Obokata papers are replicable. Can adult cells – lymphocytes, skin fibroblasts or other cells – be converted into embryonic-like stem cells by an acid stress? If yes, then this will make stem cell generation far easier and it will open up a whole new field of inquiry, leading to many new exciting questions. Do human cells also respond to acid stress in the same manner as the mouse cells? How does acid stress reprogram the adult cells? Is there an acid-stress signal that directly acts on stem cell transcription factors or does the stress merely activate global epigenetic switches? Are other stressors equally effective? Does this kind of reprogramming occur in our bodies in response to an injury such as low oxygen or inflammation because these kinds of injuries can transiently create an acidic environment in our tissues?
Researchers all around the world are currently attempting to test the effect of acid exposure on the activation of stem cell genes. Paul Knoepfler’s stem cell blog is currently soliciting input from researchers trying to replicate the work. Paul makes it very clear that this is an informal exchange of ideas so that researchers can learn from each other on a “real-time” basis. It is an opportunity to find out about how colleagues are progressing without having to wait for 6-12 months for the next big stem cell meeting or the publication of a paper confirming or denying the replication of acid-induced reprogramming. Posting one’s summary of results on a blog is not as rigorous as publishing a peer-reviewed paper with all the necessary methodological details, but it can at least provide some clues as to whether some or all of the results in the controversial Obokata papers can be replicated.
If the preliminary findings of multiple labs posted on the blog indicate that lymphocytes or skin cells begin to activate their stem cell gene signature after acid stress, then we at least know that this is a project which merits further investigation and researchers will be more willing to invest valuable time and resources to conduct additional replication experiments. On the other hand, if nearly all the researchers post negative results on the blog, then it is probably not a good investment of resources to spend the next year or so trying to replicate the results.
It does not hurt to have one’s paradigms or ideas challenged by new scientific papers as long as we realize that paradigm-challenging papers need to be replicated. The Nature papers must have undergone rigorous peer review before their publication, but scientific peer review does not involve checking replicability of the results. Peer reviewers focus on assessing the internal logic, experimental design, novelty, significance and validity of the conclusions based on the presented data. The crucial step of replicability testing occurs in the post-publication phase. The post-publication exchange of results on scientific blogs by independent research labs is an opportunity to crowd-source replicability testing and thus accelerate the scientific authentication process. Irrespective of whether or not the attempts to replicate acid-induced reprogramming succeed, the willingness of the stem cell community to engage in a dialogue using scientific blogs and evaluate replicability is an important step forward.
![]()
Obokata H, Wakayama T, Sasai Y, Kojima K, Vacanti MP, Niwa H, Yamato M, & Vacanti CA (2014). Stimulus-triggered fate conversion of somatic cells into pluripotency. Nature, 505 (7485), 641-7 PMID: 24476887