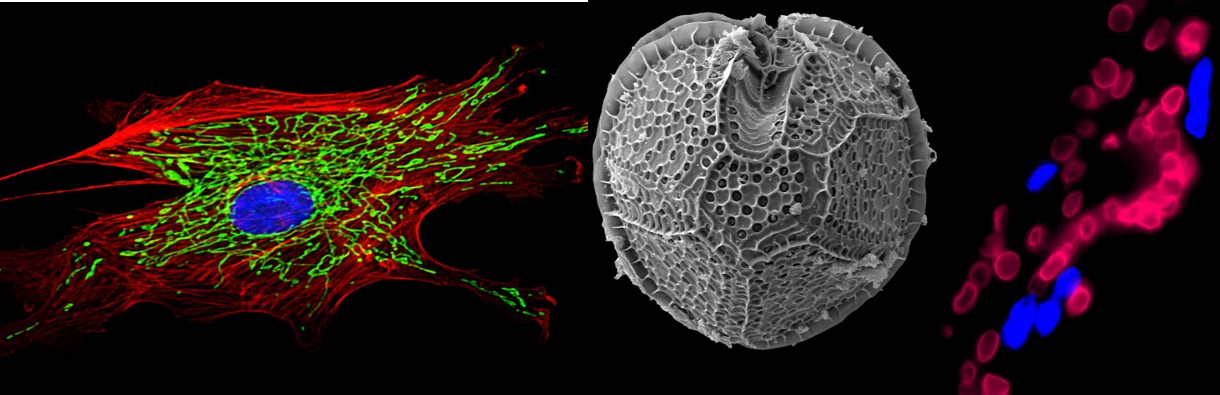
Aeon Magazine recently published my longform essay on our research with human liposuction samples and our attempts to use fat for regenerative and therapeutic purposes. Many research groups, including our own group, have been able to isolate stem cells from human fat. However, when it came to using this cells for treating cardiovascular disease, the cells behaved in a manner that we had not anticipated.

We were unable to convert them into heart muscle cells or blood vessel endothelial cells, but we found that they could help build large networks of blood vessels by releasing important growth factors. Within a few years of our initial publication, clinical trials with patients with blocked arteries or legs were already being planned, and are currently underway.
We decided to call the cells “adipose stromal cells” because we wanted to emphasize that they were acting as a “stroma” (i.e. supportive environment for blood vessels) and not necessarily as stem cells (i.e. cells that convert from an undifferentiated state into mature cell types). In other contexts, these same cells were indeed able to act like “stem cells”, because they could be converted into bone-forming or cartilage-forming cells, thus showing the enormous versatility and value of the cells that reside within our fat tissues.
The answer to the question “Does Human Fat Contain Stem Cells?” is Yes, but these cells cannot be converted into all desired tissues. Instead, they have important supportive functions that can be used to engineer new blood vessels, which is a critical step in organ engineering.
In addition to describing our scientific work, the essay also mentions the vagaries of research, the frustrations I had as a postdoctoral fellow when my results were not turning out as I had expected, and how some predatory private clinics are already marketing “fat-derived stem cell therapies” to paying customers, even though the clinical results are still rather preliminary.
For the readers who want to dig a bit deeper, here are some references and links:
1. The original paper by Patricia Zuk and colleagues which described the presence of stem cells in human liposuction fat:
Zuk, P et al (2001) “Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies”
2. Our work on how the cells can help grow blood vessels by releasing proteins:
Rehman, J et al (2004) “Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells”
3. Preliminary findings from ongoing clinical studies in which heart attack patients receive infusions of fat derived cells into their hearts to improve heart function and blood flow to the heart:
Houtgraf, J et al (2012) “First Experience in Humans Using Adipose Tissue–Derived Regenerative Cells in the Treatment of Patients With ST-Segment Elevation Myocardial Infarction”
4. Preliminary results from an ongoing trial using the fat-derived cells in patients with severe blockages of leg arteries:
Bura, A et al (2014) “Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia”
5. Example of how “cell therapies” (in this case bone marrow cells) are sometimes marketed as “stem cells” but hardly contain any stem cells:
The Largest Cell Therapy Trial in Heart Attack Patients Uses Hardly Any Stem Cells
6. The major scientific society devoted to studying the science of fat and its cells as novel therapies is called International Federation for Adipose Therapeutics and Science (IFATS).
I am not kidding, it is I-FATS!
Explore their website if you want to learn about all the exciting new research with fat derived cells.
7. Some of our newer work on how bone marrow mesenchymal stem cells turn into fat cells and what role their metabolism plays during this process:
Zhang, Y et al (2013) “Mitochondrial Respiration Regulates Adipogenic Differentiation of Human Mesenchymal Stem Cells”
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, & Hedrick MH (2001). Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue engineering, 7 (2), 211-28 PMID: 11304456
![]()
Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, & March KL (2004). Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation, 109 (10), 1292-8 PMID: 14993122